
Chimeric antigen receptor (CAR) constructs are purpose-built transmembrane proteins artificially added to immune cells, principally T cells, to enhance their ability to find and fight cancer cells. Their extracellular domains are designed to detect specific tumor-associated antigens, while their intracellular components raise the alarm within the cell when the target is encountered. However, as an iceberg that hides most of its weight beneath the surface, it is the molecular machinery that extends inwards beneath the cell membrane where CAR engineering efforts have seen the greatest advancements.. After several CAR iterations, “generations,” CAR-T cells have become longer-lived, more proliferative, more effective cancer cell killers, and less hazardous to the cancer patient. The optimization process continues to progress, and that process itself may now be undergoing an upgrade as well.
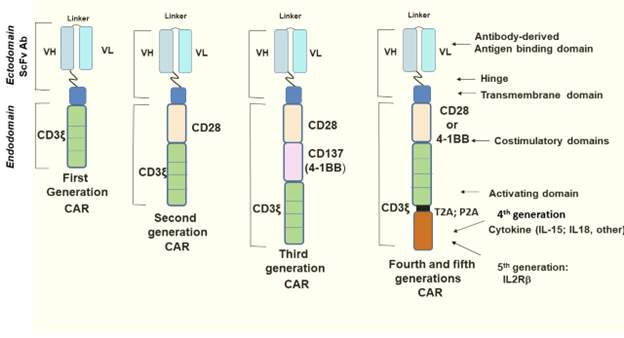
Figure 1. Evolution of CAR construct designs. Golubovskaya, 2022.
The single intracellular component of most of the first-generation CARs was CD3ζ[2]. While this basic design successfully initiated intracellular signaling events, the cell proliferation necessary for a sustained presence in the patient’s body requires a secondary signaling domain[3]. An additional co-stimulatory domain like CD28 or 4-1BB[1,4] was added in second generation CARs, with multiple co-stimulatory domains seen in the third generation. These act to amplify the CD3ζ signal and to activate other biochemical pathways that promote proliferation and ameliorate T cell exhaustion[5,6]. Fourth-generation CARs, known as T cells redirected for universal cytokine-mediated killing (TRUCK), add a cytokine component like interleukin 12 (IL-12) to the second-generation designs[7]. TRUCKs can kill tumor cells without necessarily making contact with them, by recruiting natural killer cells and other T cells to the area, and via the cytolytic action of interleukins like IL-12[7]. Lastly, fifth-generation CARs introduce an IL-2Rβ chain fragment to the second-generation template to activate the JAK-STAT pathway, further enhancing proliferation and survival[8].
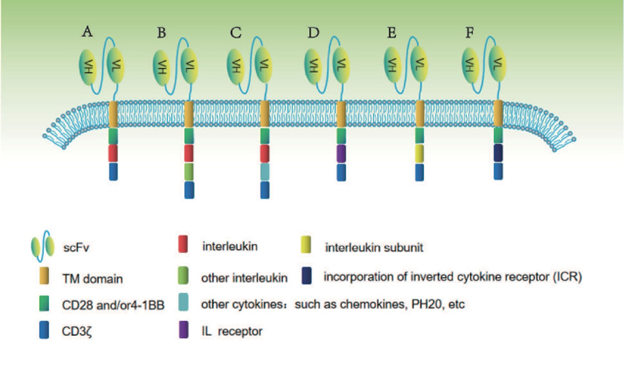
Figure 2. Fourth and fifth-generation CAR construct designs. Zhang, Miao, Ren, et al. 2021.
The CAR design advances seen in the intracellular components alone are impressive and are accelerating. Researchers are engaged in an ongoing hunt for new and better CAR components and arrangements to impart immune cells with additional weapons and strategies. However, that search itself may be about to change. A method for pooling CAR component candidates and assessing their effectiveness in a multiplexed fashion was recently published[10]. The study identified promising novel domains and strategies for the next generation of CAR designs, pointing the way toward advancements in the challenging arena of solid tumor immunotherapies.
References
- Vita Golubovskaya. CAR-T Cells Targeting Immune Checkpoint Pathway Players. Front. Biosci. (Landmark Ed) 2022, 27(4), 121. https://doi.org/10.31083/j.fbl2704121
- Irving, B.A., Weiss, A. The cytoplasmic domain of the T cell receptor ζ chain is sufficient to couple to receptor-associated signal transduction pathways. Vol 64, Issue 5, p891-901, MARCH 08, 1991. https://doi.org/10.1016/0092-8674(91)90314-O
- Gimmi, C.D, Freeman, G.J., Gribben, J.G., et al. Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc. Natl. Acad. Sci. Vol. 90, pp. 6586-6590, July 1993. Immunology. https://pnas.org/doi/pdf/10.1073/pnas.90.14.6586
- Feins, S., Kong, W., Williams, E.F., et al. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Amer. Journal Hematology, Vol 94, Iss S1, Special Issue: CAR T Cells in Cancer Therapy. May 2019. pp S3-S9. https://doi.org/10.1002/ajh.25418
- Long, A.H., Haso, W.M., Shern, J.F., et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015 Jun;21(6):581-90. https://doi.org/10.1038/nm.3838
- Esensten, J.H., Helou, Y.A., Chopra, G., et al. CD28 Costimulation: From Mechanism to Therapy. Immunity, Vol 44, Iss 5, 2016, pp 973-988, ISSN 1074-7613, https://doi.org/10.1016/j.immuni.2016.04.020
- Zhang, C., Liu, J., Zhong, J.F. et al. Engineering CAR-T cells. Biomark Res 5, 22 (2017). https://doi.org/10.1186/s40364-017-0102-y
- Tokarew, N., Ogonek, J., Endres, S. et al. Teaching an old dog new tricks: next-generation CAR T cells. Br J Cancer 120, 26–37 (2019). https://doi.org/10.1038/s41416-018-0325-1
- Zhang, Z., Miao, L., Ren, Z., et al. Gene-Edited Interleukin CAR-T Cells Therapy in the Treatment of Malignancies: Present and Future. Front. Immunol., 27 July 2021. Sec. Cancer Immunity and Immunotherapy. Vol 12 – 2021. https://doi.org/10.3389/fimmu.2021.718686
- Goodman, D.B., Azimi, C.S., Kearns, K., et al. Pooled screening of CAR T cells identifies diverse immune signaling domains for next-generation immunotherapies. Science Translational Medicine. 2022 Nov;14(670):eabm1463. https://doi.org/10.1126/scitranslmed.abm1463
