A variety of DNA modification tools are relied upon for advanced gene and cell therapies (i.e. CAR-T cells) to continue to progress. These include examples like CRISPR-based systems and viral vectors. However, the underlying bottleneck for many of these methods is high-purity plasmid DNA (pDNA) and current market supply is struggling to keep up with demand.
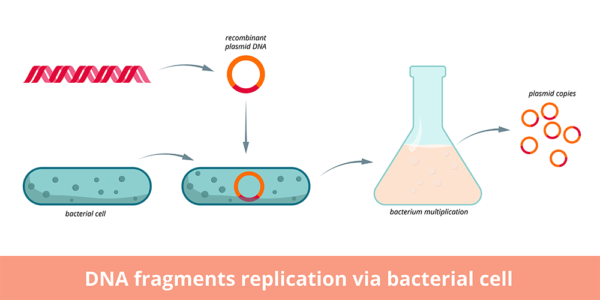
Large-scale plasmid production begins with transformation of E. coli bacteria with a small amount of starting plasmid containing desired sequences, often including selection genes which confer fluorescence or antibiotic resistance, as well as transgenes for downstream transfection applications. Two principle techniques for carrying out the initial transformation include heat shock and electroporation. Successfully transformed bacteria can be selected for by culturing them on media containing the antibiotic to which the plasmid conferred resistance, or by visual inspection to identify fluorescence resulting from fluorescent proteins encoded on the plasmid. Transformed bacterial cultures are transferred to fresh media for further cultivation.
Selected E. coli are fermented in sequentially larger volumes in sterile flasks using aseptic technique. Optical density measurements of samples of the culture are taken to monitor where the culture is along the anticipated growth curve. Sequential expansion can be undertaken in larger and larger containers. Once the culture has the desired optical density, centrifugation is used to collect the bacteria into a cell pellet and the used media is discarded.
The cell pellet can then be resuspended and chemically lysed. Debris filtration, sequential centrifugation steps and chemical purification can be employed to rid the solution of cell debris, leaving behind clarified lysate containing the desired plasmids. A UV/Vis spectrophotometer can be used to assess pDNA content.
