The high efficacy of some adoptive immune therapies, primarily those based on CAR-T and also CAR-NK cells is rather incredible in the face of the numerous barriers in the way to success. Creating the therapy in the first place requires a sufficient supply of unmodified cells in good condition. This can be especially difficult in the case of patients who have already undergone harsh cancer treatments. The subsequent process of introducing a chimeric antigen receptor (CAR) construct is highly complex. It involves knocking out the functions of specific genes, introducing new ones, the CAR construct itself, and doing so at genomic loci which may need to be chosen using advanced predictive bioinformatics tools. Target sites may feature critical nearby regulatory elements. Conversely, off-target insertions can trigger genomic instability, cell death, or even the beginnings of a future cancer. All this just gets the therapy to the starting line: infusion. After administration into the patient’s body, are the cells completely on their own, never to be seen again? Not quite. Researchers have developed a number of techniques for tracking the modified cells within the body, and optimization efforts are ongoing. Here, we will discuss some methods that have been developed in this important facet of the adoptive immunotherapy field.
Firstly, perhaps it is also worth asking what there is to gain from this. Once the therapy has been administered, why not just monitor the patient’s health? Certainly, the efficacy of the cell product may be measured by just following the progression of the disease. However, answers to why the therapy may fail in one patient and bring another patient to full remission might lie precisely in seeing what the cells did once they entered the patient’s body. Did they exhibit rapid exhaustion? Did they accumulate at the tumor site at all? Did they accumulate elsewhere? There is a potential treasure trove of information to be learned by looking, and there are now a few ways to do that.
Radiolabeling, adding small amounts of a radioactive substance to the modified cells prior to infusion, is one relatively straightforward way to enable visualization. There is no specific metabolic pathway that must take place in this approach, no exogenous gene that must be inserted. For example, inclusion of substances containing Zirconium-89 or Indium-111 can subsequently be visualized by positron emission tomography (PET) and single-photon emission computed tomography (SPECT), respectively.
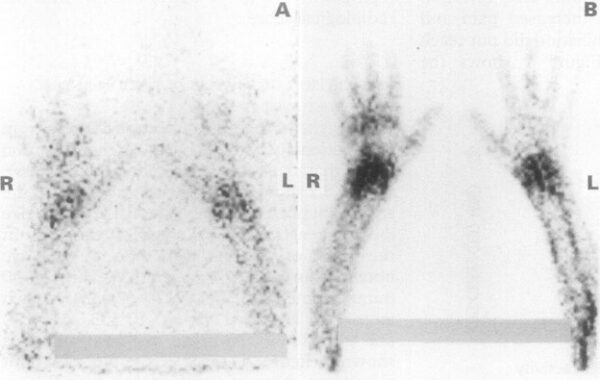
Radio-labelled lymphocyte scintigram of a patient with RA and wrist arthritis. A: Images at 30 minutes showing early lymphocyte accumulation in the inflamed joints; B: same patient at three hours. Source: Jorgensen, 1995.
The reporter-gene strategy adds one layer of complexity over simple radiolabeling. In this case, a new gene is indeed introduced into the cell. The cell expresses the reporter gene product which will then bind to a probe molecule. That probe molecule can bring with it a detectable radioactive atom. However, there are several advantages to this approach. Firstly, dead cells are not metabolically active and stop expressing the reporter gene. This means any probe signal conveys not just the location of an edited cell, but also the fact that it is alive. Secondly, the radioisotope used can be a more short-lived one, such as Gallium-68 or Fluorine-18, whose half-lives are less than two hours. This reduces the amount of time precious CAR-T cells must be in the vicinity of a radioactive substance.
While radiolabeling is not a new tool in biology, its application as part of the advances being seen in cutting-edge immunotherapy research adds an exciting new layer in an already dynamic field. The future of adoptive immunotherapy looks promising, all the more so because we may soon get to see it with our own eyes.
References
- Jorgensen, C., Couret, I., Bologna, C., Rossi, M., & Sany, J. (1995). Radiolabeled lymphocyte migration in rheumatoid synovitis. Annals of the Rheumatic Diseases, 54(1), 39-44. https://doi.org/10.1136/ard.54.1.39
- Shao, F., Long, Y., Ji, H., Jiang, D., Lei, P., & Lan, X. (2021). Radionuclide-based molecular imaging allows CAR-T cellular visualization and therapeutic monitoring. Theranostics, 11(14), 6800-6817. https://doi.org/10.7150/thno.56989
- Shalaby, N., Xia, Y., Kelly, J., et al. (2024). Imaging CAR-NK cells targeted to HER2 ovarian cancer with human sodium-iodide symporter-based positron emission tomography. European Journal of Nuclear Medicine and Molecular Imaging. Advance online publication. https://doi.org/10.1007/s00259-024-06722-w
- Skovgard, M. S., Hocine, H. R., Saini, J. K., Moroz, M., Bellis, R. Y., Banerjee, S., Morello, A., Ponomarev, V., Villena-Vargas, J., & Adusumilli, P. S. (2021). Imaging CAR T-cell kinetics in solid tumors: Translational implications. Molecular Therapy Oncolytics, 22, 355-367. https://doi.org/10.1016/j.omto.2021.06.006
